B. Increased dose of inhaled corticosteroid – Explanation
at step 4 of the BTS guideline which recommends increasing the ICS dose to 800
mcg/day or to consider a trial of Theophylline. Be mindful that children and adult
recommendations are different
ASTHMA STEP UP MANAGEMENT ACCORDING TO SIGN/BTS 2016

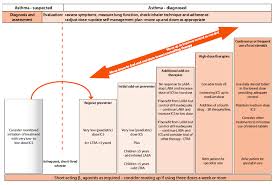
ASTHMA NICE VS BTS/SIGN GUIDELINES FOR CHILDREN
Asthma is characterised by paroxysmal and reversible obstruction of the airways.
Stepwise approach if asthma remains uncontrolled
| NICE 2017 | SIGN/BTS 2016 |
|---|---|
| Step 1 Inhaled short-acting B2 agonist (SABA)as required |
Step 1 Inhaled short-acting B2 agonist (SABA)as required |
| Step 2 Add paediatric low dose inhaled corticosteroid (ICS) upto 200 mcg/day |
Step 2 Add paediatric very low dose inhaled corticosteroid (ICS) up to 200 mcg/day |
| Step 3 Add leukotriene receptor antagonist (LTRA) Review response in 4-8 weeks |
Step 3 |
|
Step 4 Stop LTRA and start inhaled long- combination with paediatric low dose |
Step 4 Consider trials of – Increasing inhaled steroid up to 8000 mcg/day – Add SR theophylline |
| Step 5 Stop LABA and ICS and start MART* regimen with a paediatric low dose ICS |
Step 5 Use daily steroid tablets |
| Step 6 Increase to paediatric moderate maintenance dose ICS (either continuing MART or changing to fixed dose ICS + LABA and SABA as reliever |
|
| Step 7 Refer to a respiratory physician for further escalation |
*Maintenance and reliever therapy (MART) is a form of combined ICS and LABA
treatment in which a single inhaler, containing both ICS and a fast-acting LABA,
is used for both daily maintenance therapy and the relief of symptoms as
required. The use of SABA is not needed when on MART.
Under 5 years
| NICE 2017 | SIGN/BTS 2016 |
|---|---|
| Step 1 Inhaled short-acting B2 agonist (SABA)as required |
Step 1 Inhaled short-acting B2 agonist (SABA)as required |
| Step 2 Add paediatric low dose inhaled corticosteroid (ICS) upto 400 mcg/day on an 8-week trial |
Step 2 Add inhaled corticosteroid (ICS) at very low paediatric dose of 200 mcg/day but consider leukotriene receptor antagonist (LTRA) who are unable to take ICS |
| Step 3 After 8 weeks stop ICS treatment and monitor symptoms. If symptoms – Did not resolve during the trial period, review likelihood of alternative diagnosis – Resolved but re-occurred beyond 4 weeks after stopping ICS treatment, repeat the 8-week trial of a paediatric moderate dose of ICS – Resolved then re-occurred within 4 weeks of stopping ICS treatment, restart the ICS at a paediatric low dose as first-line maintenance therapy and see Step 4 |
Step 3 Add leukotriene receptor antagonist (LTRA) for under 5 years if not already on this |
| Step 4 Add leukotriene receptor antagonist (LTRA) |
Step 4 Refer to a respiratory physician for further management |
| Step 5 Refer to a respiratory physician for further management |
